Sermorelin
$35.00
Free Bacteriostatic Water 30ml
with all orders!Free Bacteriostatic Water 30ml
with all orders!
Free Shipping to continental US
for orders over $200Free Shipping to continental US
for orders over $200Sermorelin is a growth-hormone-releasing hormone (GHRH) analogue used clinically to assess growth hormone secretion. It is of interest to researchers for its ability to improve bone density, reduce scaring, fight the effects of dementia, and reduce seizure activity.
| Weight | 2 g |
|---|---|
| Dimensions | 5 × 10 × 12 cm |
What is Sermorelin?
Sermorelin is one of a handful of growth hormone releasing hormone (GHRH) analogues that have been developed in recent years in an effort to preserve some of the positive effects of natural GHRH while avoiding undesirable effects. Sermorelin (Geref) is currently used clinically to assess growth hormone secretion, but the peptide is of additional interest for its abilities to:
- reduce scarring following heart attack,
- increase bone density,
- improve nutrition in chronic illness,
- improve renal function,
- fight the effects of dementia, and
- reduce seizure activity.
Sermorelin Structure
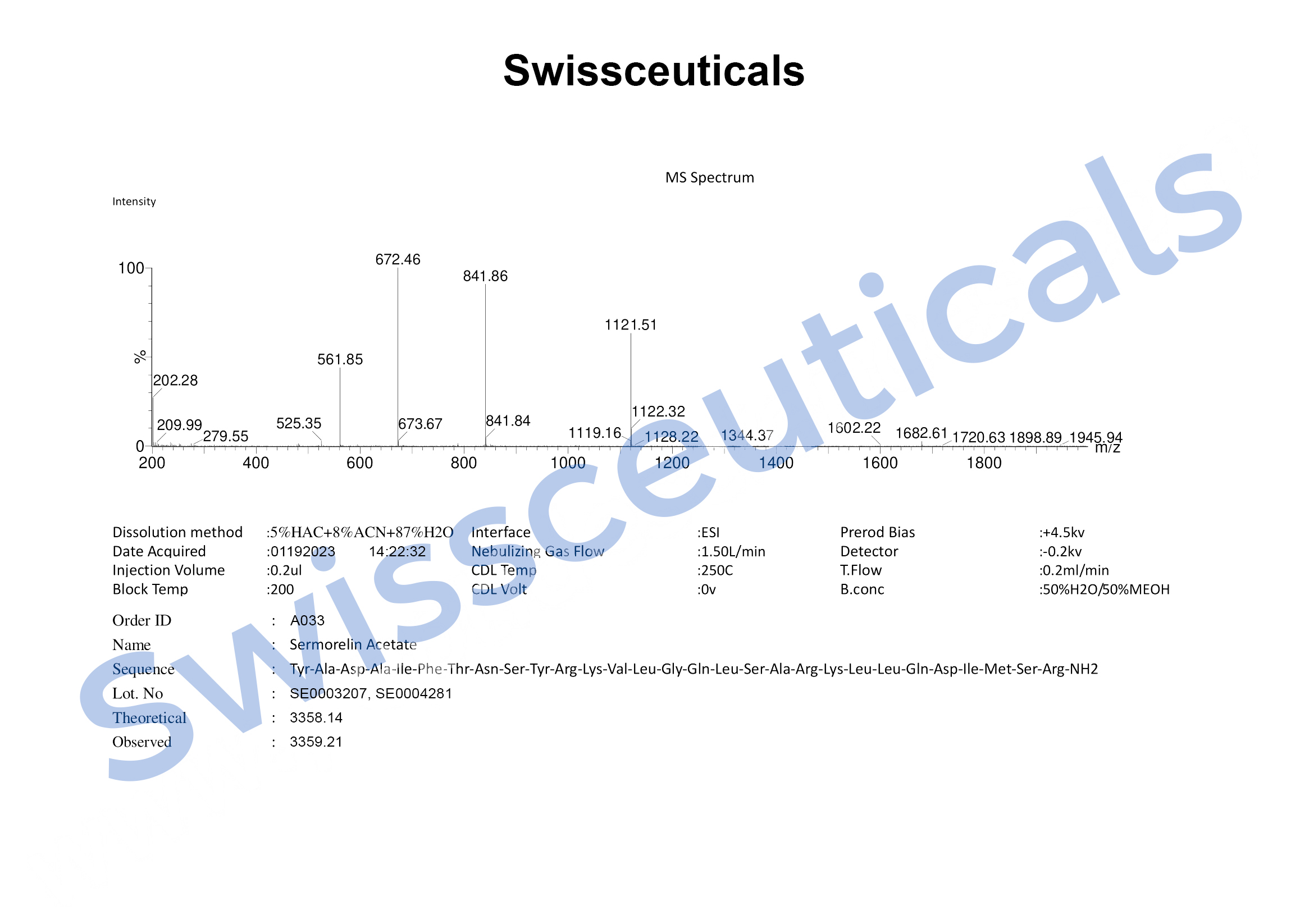
Sequence: Tyr-DL-Ala-DL-Asp-DL-Ala-DL-xiIle-DL-Phe-DL-xiThr-DL-Asn-DL-Ser-DL-Tyr-DL-Arg-DL-Lys-DL-Val-DL-Leu-Gly-DL-Gln-DL-Leu-DL-Ser-DL-Ala-DL-Arg-DL-Lys-DL-Leu-DL-Leu-DL-Gln-DL-Asp-DL-xiIle-DL-Met-DL-Ser-DL-Arg
Molecular Formula: C149H246N44O42S
Molecular Weight: 3357.933 g/mol
PubChem CID: 16129620
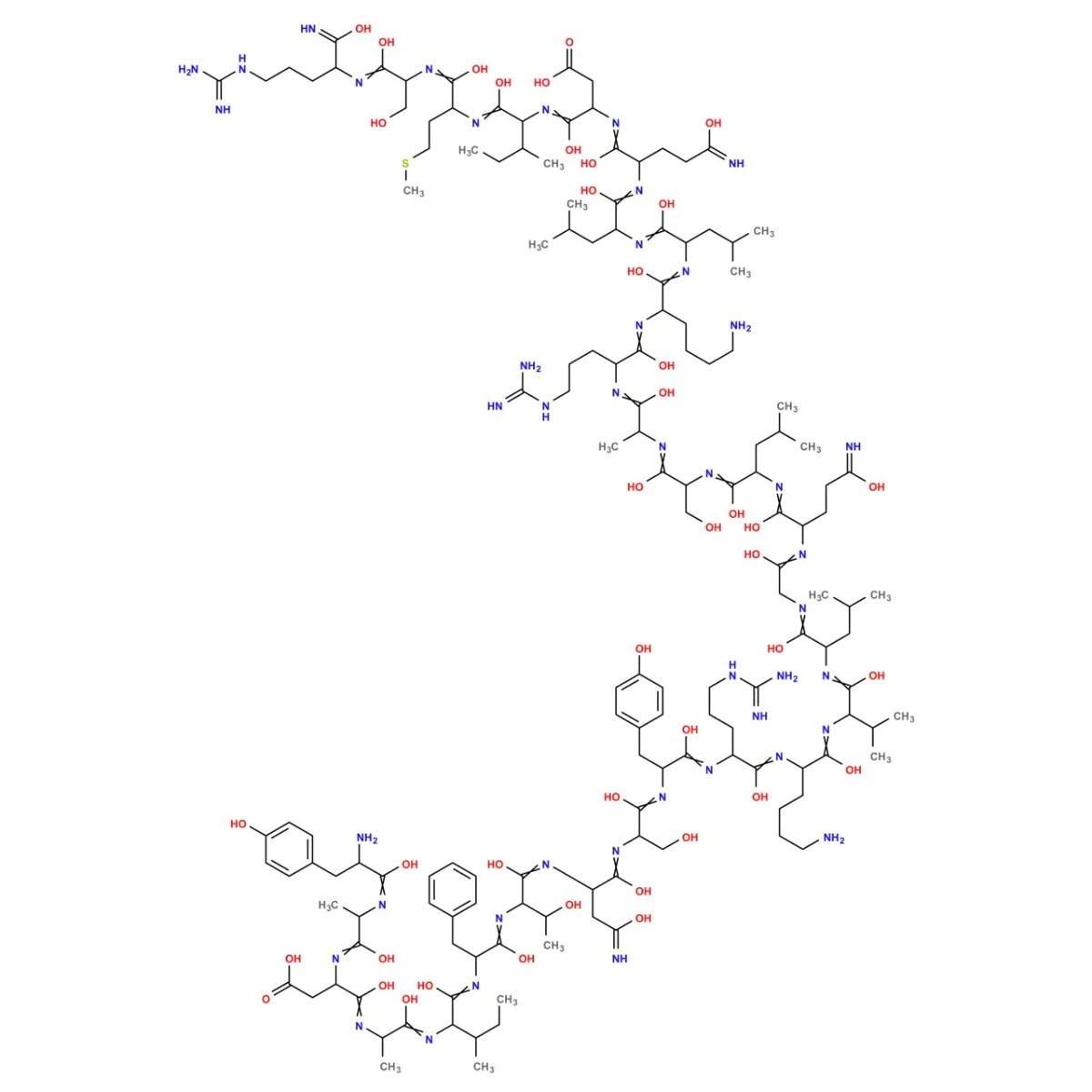
Source: PubChem
Sermorelin Peptide Research
Sermorelin and Heart Health
Heart attack, while acutely life-threatening, can also lead to long-term disability secondary to heart failure, cardiac conduction abnormalities (arrhythmias), reduced exercise capacity, pain, and more. A number of these problems result from cardiac remodeling that follows damage to myocytes (heart muscle cells). Often, cardiac remodeling leads not only to scarring in the area of damage following a heart attack, but in surrounding, undamaged areas as well. This remodeling causes a number of long-term problems and research has shown that preventing it from happening can significantly improve outcomes both immediately after heart attack and years down the line.
In 2016, a study in pigs revealed that sermorelin administration is effective in reducing the remodeling that follows a heart attack. The research showed that sermorelin:
- reduces cell death in cardiomyocytes,
- increases the production of extracellular matrix components needed for adequate healing,
- increases the growth of blood vessels to damaged tissue, and
- reduces the production of substances that causes damaging inflammation.
Clinically, sermorelin’s effects are seen in improved diastolic function, reduced scar size, and increased capillary growth[1], [2]. There is current research exploring the benefits of sermorelin in other forms of heart disease, such as heart failure and even valve disorders.
GHRH treatment reduces scar mass. A. Shows graph of percent change in scar mass over time on top and the relationship between the percent change in scar mass as a percentage of left ventricular mass. B. Shows images of the heart before and after 4 weeks of sermorlin treatment or placebo.
Sermorelin and Epilepsy
Gamma-aminobutyric acid (GABA) is a central nervous system signaling molecule known to reduce electrical activity in the spinal cord and reduce overall electrical excitability in the central nervous system. A number of anti-seizure medications work either by increasing levels of GABA in the central nervous system or by binding to GABA receptors and mimicking the effects of GABA. In a recent study of mice with epilepsy, scientists administered GHRH analogues, like sermorelin, to test the effect of these peptides on seizure activity. It turns out that GHRH analogues are effective in suppressing seizures by activating GABA receptors[3]. This is a very new finding and an active area of research as medications for treating seizure conditions, while effective, have a range of detrimental side effects that reduce their clinical use.
Sermorelin and Sleep
There is good evidence that sleep cycles are regulated by orexin, a potent neurochemical produced by certain neurons in the brain. It is also well understood that growth and healing, which are strongly associated with growth hormone secretion, primarily take place during sleep. Research in rainbow trout suggests that this is no coincidence, with an intact GHRH axis being a necessary component for proper orexin secretion and function. In addition, the research reveals that exogenous administration of sermorelin and other GHRH agonists can boost orexin secretion [4]. There is ongoing research into the benefits of using sermorelin in sleep disorders.
Sermorelin Preferred to Growth Hormone
Sermorelin is a growth hormone releasing hormone derivative and, as such, produces all of the same effects that GH produces, including increasing muscle mass, boosting long bone growth, and reducing adipose tissue. Even though the effects are the same, the side effects are not. In fact, sermorelin is the preferred way to increase GH levels in humans, even over the exogenous administration of growth hormone itself. The primary reason for this preference is that sermorelin is subject to physiological feedback mechanisms that help to prevent common problems encountered with GH administration. These problems include overdose, improper dosing, and unintended side effects like edema, joint pain, and dysregulation of normal physiology[5].
A second reason to prefer sermorelin is that research shows it is not subject to tachyphylaxis, the process by which the body becomes accustomed to a medication and requires higher and higher doses to achieve desired effects. In some cases, tachyphylaxis is so severe that a drug holiday (complete cessation of use of a medication) is required to regain the effects of a medication. Long-term use of sermorelin in certain clinical settings as well as animal studies of the peptide indicate that the body has a unique response to the peptide. Rather than down-regulate the production of GHRH receptors with administration of sermorelin, the body instead increases their production. This ensures that sermorelin’s effects are unchanged, that tachyphylaxis does not develop to a substantial degree, and that dose escalation is generally not required[6].
Sermorelin exhibits moderate side effects, low oral and excellent subcutaneous bioavailability in mice. Per kg dosage in mice does not scale to humans. Sermorelin for sale at Peptide Sciences is limited to educational and scientific research only, not for human consumption. Only buy Sermorelin if you are a licensed researcher.
Article Author
The above literature was researched, edited and organized by Dr. Logan, M.D. Dr. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
Scientific Journal Author
Richard F. Walker, Ph.D, R.Ph, lead author of A better approach to management of adult-onset growth hormone insufficiency?”, received a BS in pharmacy from Rutgers University, a MS in Biochemistry from New Mexico State University and a PhD in a physiology from Rutgers University. He holds postdoctoral fellowships in neuroendocrinology and neuropharmacology at Duke University College of Medicine (Center for the Study of Aging and Human Development) and the University of California, Berkeley, respectively.
Richard F. Walker, Ph.D, R.Ph is being referenced as one of the leading scientists involved in the research and development of Sermorelin. In no way is this doctor/scientist endorsing or advocating the purchase, sale, or use of this product for any reason. There is no affiliation or relationship, implied or otherwise, between Peptide Sciences and this doctor. The purpose of citing the doctor is to acknowledge, recognize, and credit the exhaustive research and development efforts conducted by the scientists studying this peptide. Richard F. Walker, Ph.D, R.Ph is listed in [5] under the referenced citations.
Referenced Citations
- L. L. Bagno et al., “Growth Hormone–Releasing Hormone Agonists Reduce Myocardial Infarct Scar in Swine With Subacute Ischemic Cardiomyopathy,” J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis., vol. 4, no. 4, Mar. 2015.
- R. M. Kanashiro-Takeuchi et al., “New therapeutic approach to heart failure due to myocardial infarction based on targeting growth hormone-releasing hormone receptor,” Oncotarget, vol. 6, no. 12, pp. 9728–9739, Mar. 2015.
- S. Tang et al., “Interactions between GHRH and GABAARs in the brains of patients with epilepsy and in animal models of epilepsy,” Sci. Rep., vol. 7, Dec. 2017.
- B. S. Shepherd et al., “Endocrine and orexigenic actions of growth hormone secretagogues in rainbow trout (Oncorhynchus mykiss),” Comp. Biochem. Physiol. A. Mol. Integr. Physiol., vol. 146, no. 3, pp. 390–399, Mar. 2007.
- R. F. Walker, “Sermorelin: A better approach to management of adult-onset growth hormone insufficiency?,” Clin. Interv. Aging, vol. 1, no. 4, pp. 307–308, Dec. 2006.
- S. T. Wahid, P. Marbach, B. Stolz, M. Miller, R. A. James, and S. G. Ball, “Partial tachyphylaxis to somatostatin (SST) analogues in a patient with acromegaly: the role of SST receptor desensitisation and circulating antibodies to SST analogues,” Eur. J. Endocrinol., vol. 146, no. 3, pp. 295–302, Mar. 2002.
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Certificate of Analysis (COA)
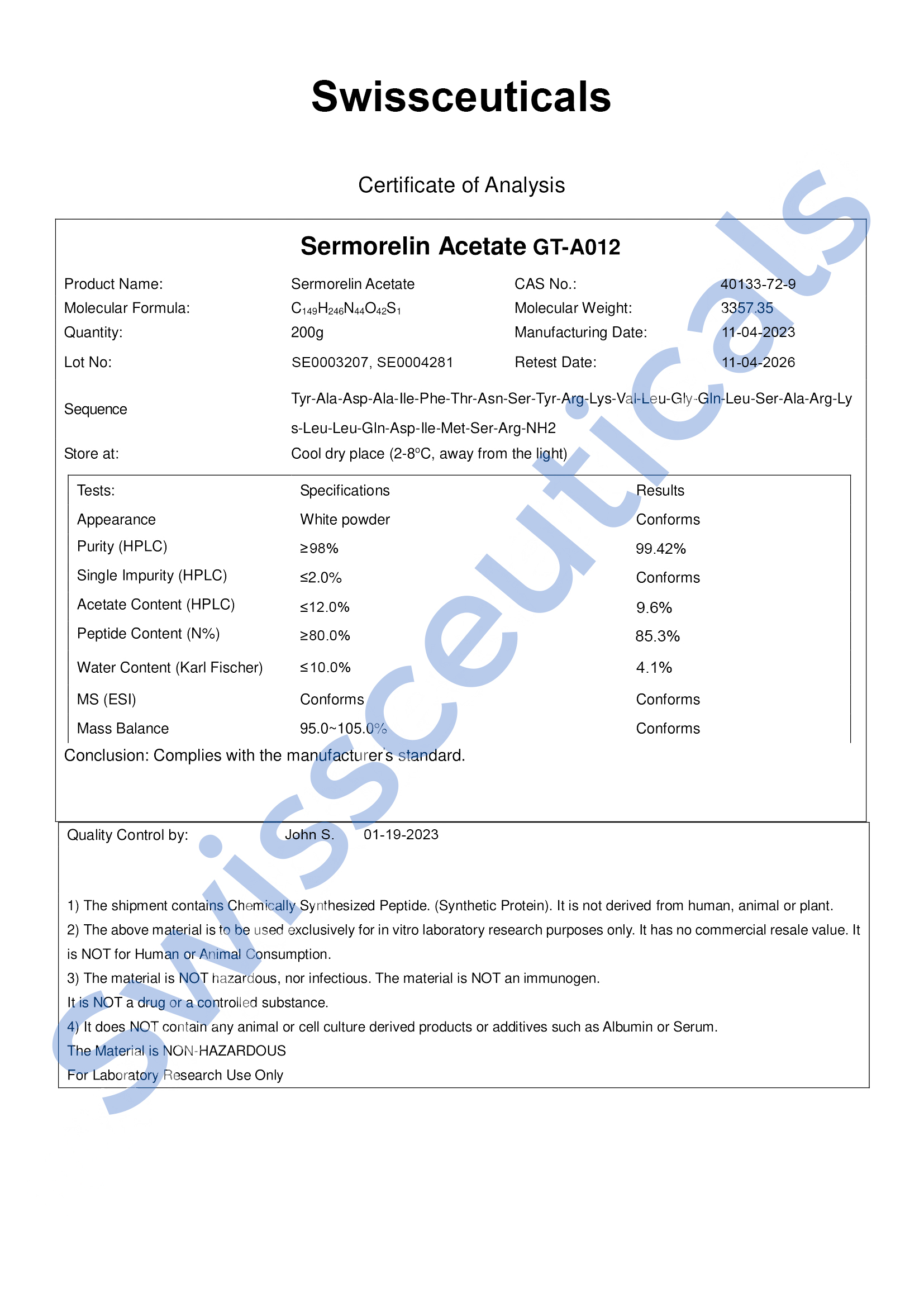
High Performance Liquid Chromatography (HPLC)
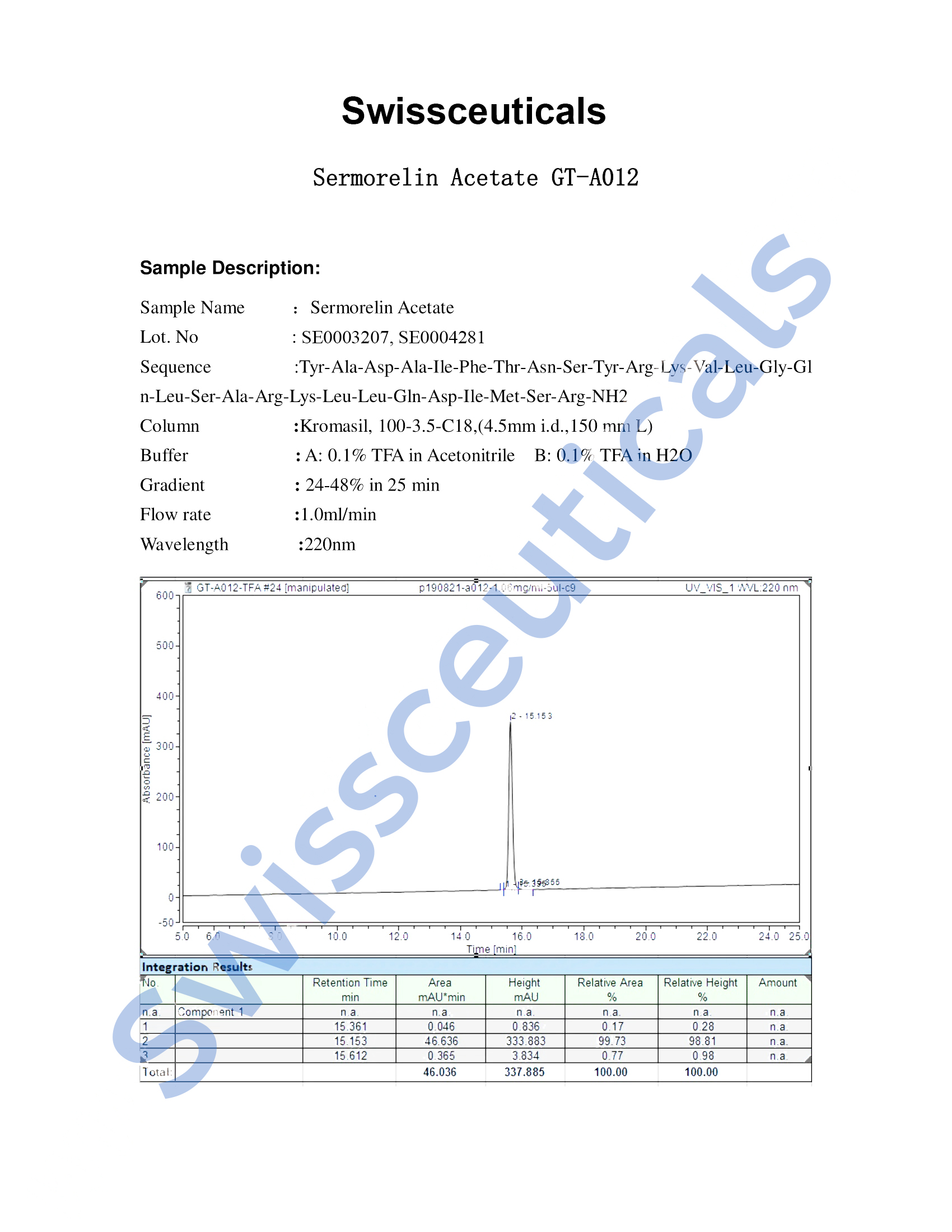
Mass Spectrometry (MS)