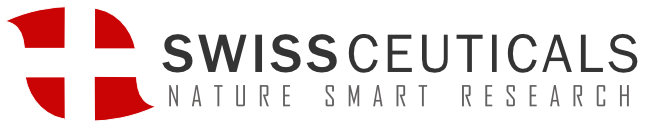ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Cognition is a complex system encompassing processes such as episodic memory, working memory, executive function/inhibition, spatial learning, language/vocabulary comprehension, processing speed, and language/reading decoding. Changes in synaptic plasticity, the ability of the brain to change and adapt to new information, can be short lived from milliseconds to years. Short lived forms include facilitation, augmentation, and potentiation which enhances neurotransmitter release.
These dynamic changes represent the molecular basis for learning and memory. This synaptic plasticity can be influenced by several factors e.g., aging, diseases (obesity, diabetes, hypertension, dyslipidemia), toxins (smoking and alcohol), and exercise. Aging has been estimated to trigger performance decline with an incidence of mild cognitive impairment of 21.5–71.3 per 1000 person-years). Cortical thickness and subcortical volume are shrinking 0.5–1% annually as a morphological sign of cognitive decline with plaques and axonal degeneration. Dementia is diagnosed when the acquired cognitive impairment has become severe enough to compromise social and/or occupational functioning with increasing prevalence.
Worldwide, around 50 million people have dementia and, with one new case every three seconds, the number of people with dementia is set to triple by 2050. Thus, there is a huge need for new research in order to combat the above-mentioned metrics. The peptides below have undergone extensive research to help aid in the improvement for our neurocognitive system.
Selank
Both Selank and Semax are melanocortin’s and have pleiotropic effects involved in brain health and function. Selank by itself has traditionally been prescribed for anxiety and depression. Selank has pronounced anxiolytic activity and acts as a stable neuropsychotropic, antidepressant, and anti-stress medication.
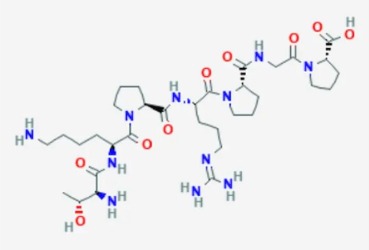
Semax
Semax is used as a therapeutic with pathologies related to brain circulation dysfunction. As a combination, Selank/Semax has applications in improving learning processes, exploratory behavior, regeneration and development, nociceptive and in amatory processes, accelerate nerve regeneration and improve neuromuscular performance and overall neural health.
How does Selank/Semax work?
Selank and Semax act as Melanocortin Stimulating Hormone-like Peptides within the brain to initiate wide ranging reactions related to brain activity and fitness. Selank is often used for its anxiolytic properties, immune improvement, gastric protection, and with opioid and alcohol withdrawal/ dependence. Semax has been prescribed for anxiety, memory improvement, ischemic events, stroke, nerve regeneration, ADHD, opioid withdrawal and even chronic diseases such as ALS, Parkinson’s Disease, and Alzheimer’s.
Who should explore Selank/Semax?
The Selank/Semax combination works for those that are interested in improving mental clarity, memory, and focus and has applications in alleviating anxiety and depression. In addition, according to research, this medication has shown therapeutic agency with individuals suffering from PTSD and/or substance dependence withdrawal.
Indications:
- Reduces anxiety and depression
- Improves mental clarity, memory, and focus
Selank Research Example:
P-1114 – Rapid and slow response during treatment of generalized anxiety disorder with peptide anxiolytic selank
Introduction
Heptapeptide Selank approved for the treatment of Generalized Anxiety Disorder (GAD). Studies revealed that Selank acts as an anxiolytic with stimulatory and cognitive enhancing properties. Though time to treatment response significantly varied in patients and this issue has not been studied.
Individual treatment response analysis in Selank-treated patients with GAD.
To compare clinical state and EEG changes in patients with different time to treatment response to Selank.
20 patients aged 24–52 y.o. with GAD according to DSM-IV were included. Selank was administered at the dose 2700 μg/day intranasally. Study utilized valid clinical scales and pharmaco-EEG.
40% of patients were rapid responders (RR) and characterized by abrupt reduction of whole set of symptoms in the first 1–3 Days. At the Day 3 Hamilton Anxiety Rating Scale (HARS) mean total score [SD] reduced from 20.3[11.9] to 7.0[2.9] (p < 0.01). 60% of patients responded gradually (conventional responders – CR). Clinically significant changes of mean HARS total score from 16.1[7.2] to 6.2[4.7] were achieved at Day 14, p < 0.01. In contrast to CR, RR demonstrated obvious EEG-reaction after single dose (900 μg) with increase of beta-rhythm, decrease of theta- and low frequencies of alpha-rhythm (all p < 0.05). Initially RR and CR significantly differed by the score of aesthetic and cognitive symptoms (p < 0.05).
Data that peptide anxiolytic drug Selank in patients with GAD exert significant individual variability of therapeutic effect, including rapid effectiveness, confirmed. RR had more asthenic and cognitive symptoms and was characterized by a higher EEG-reactivity than CR.
Semax Research Example:
Semax, an analog of ACTH(4–10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus
The heptapeptide Semax (Met–Glu–His–Phe–Pro–Gly–Pro) is an analog of the adrenocorticotropin fragment (4–10) which after intranasal application has profound effects on learning and exerts marked neuroprotective activities. Here, we found that a single application of Semax (50 μg/kg body weight) results in a maximal 1.4-fold increase of BDNF protein levels accompanying with 1.6-fold increase of trkB tyrosine phosphorylation levels, and a 3-fold and a 2-fold increase of exon III BDNF and trkB mRNA levels, respectively, in the rat hippocampus. Semax-treated animals showed a distinct increase in the number of conditioned avoidance reactions. We suggest that Semax affects cognitive brain functions by modulating the expression and the activation of the hippocampal BDNF/trkB system.
Thymosin Beta-4
What is it?
Thymosin is a hormone secreted from the thymus. The thymus is responsible for regulating the immune system and tissue repair. Thymosin Beta-4 has been found to play an important role in protection, regeneration, and remodeling of injured or damaged tissue. It is prescribed for acute injury, surgical repair, and patients that were once athletes that accumulated injury over their lifetime. It acts as a major actin-sequestering molecule and can be taken after cardiac injury for better healing of the tissue in the heart. It is best prescribed to be taken once daily for 20 days.
How does Thymosin Beta-4 work?
Thymosin Beta-4 is typically found in both types of muscle in the human body – skeletal (the muscles that are required to move) and smooth (muscles such as those in the heart). When damage to tissue occurs, Thymosin Beta-4 is upregulated. Thymosin Beta-4 is released in the body to help people heal from traumas. In the process of healing from injury, It also acts to reduce the amount of scar tissue and improve flexibility. It also has potent anti-inflammatory properties.
- Calms muscle spasm
- Improves muscle tone
- Increases exchange of substances between cells
- Encourages tissue repair
- Helps maintain flexibility
- Reduces inflammation of tissue in joints
- Encourages growth of new blood cells in tissue
- Increases endurance and strength
- Prevents the formation of adhesions and fibrous bands in muscles, tendons, and ligaments
Indications:
- Improves healing time
- Increase strength
- Increase endurance
Thymosin Beta 4 Research Example:
Neuroprotective and neurorestorative effects of Thymosin beta 4 treatment following experimental traumatic brain injury
Traumatic brain injury (TBI) remains a leading cause of mortality and morbidity worldwide. No effective pharmacological treatments are available for TBI because all Phase II/III TBI clinical trials have failed. This highlights a compelling need to develop effective treatments for TBI. Endogenous neurorestoration occurs in the brain after TBI, including angiogenesis, neurogenesis, synaptogenesis, oligodendrogenesis and axonal remodeling, which may be associated with spontaneous functional recovery after TBI. However, the endogenous neurorestoration following TBI is limited. Treatments amplifying these neurorestorative processes may promote functional recovery after TBI. Thymosin beta4 (Tβ4) is the major G-actin-sequestering molecule in eukaryotic cells. In addition, Tβ4 has other properties including anti-apoptosis and anti-inflammation, promotion of angiogenesis, wound healing, stem/progenitor cell differentiation, and cell migration and survival, which provide the scientific foundation for the corneal, dermal, and cardiac wound repair multicenter clinical trials. Here, we describe Tβ4 as a neuroprotective and neurorestorative candidate for treatment of TBI.
Dihexa
What is it?
Dihexa is a nootropic (cognitive enhancer) and a small peptide that has been developed by researchers from Washington State University to potently improve certain cognitive function of potential trauma-based brain disorders and neurodegenerative conditions through increased synaptogenesis.
Dihexa has also been called a “neurogenic wonder-drug” and the peptide can be ten million times stronger than BDNF (Brain-derived neurotrophic factor), one of the leading medications for new synapse formation.
If you’re someone looking to enhance your mental stamina and improve your memory overall, Dihexa may be your solution to dramatically improving cognitive function.
Indications:
- Help people who have Alzheimer’s Disease (AD) and Parkinson’s Disease (PD)
- Increase mental stamina
- Enhance creative thinking and social intuition and conversational skills
- Improve problem-solving skills
- Manage depression
- Improve general long and short-term memory
- Improve focus and learning
- Improve heart health
- Improve hair health
- Boost mental endurance
Dihexa Research Example:
The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system
A subset of angiotensin IV (AngIV)-related molecules are known to possess procognitive/antidementia properties and have been considered as templates for potential therapeutics. However, this potential has not been realized because of two factors: 1) a lack of blood-brain barrier-penetrant analogs, and 2) the absence of a validated mechanism of action. The pharmacokinetic barrier has recently been overcome with the synthesis of the orally active, blood-brain barrier-permeable analog N-hexanoic-tyrosine-isoleucine-(6) aminohexanoic amide (dihexa). Therefore, the goal of this study was to elucidate the mechanism that underlies dihexa’s procognitive activity. Here, we demonstrate that dihexa binds with high affinity to hepatocyte growth factor (HGF) and both dihexa and its parent compound Norleucine 1-AngIV (Nle(1)-AngIV) induce c-Met phosphorylation in the presence of subthreshold concentrations of HGF and augment HGF-dependent cell scattering. Further, dihexa and Nle(1)-AngIV induce hippocampal spinogenesis and synaptogenesis similar to HGF itself. These actions were inhibited by an HGF antagonist and a short hairpin RNA directed at c-Met. Most importantly, the procognitive/antidementia capacity of orally delivered dihexa was blocked by an HGF antagonist delivered intracerebroventricularly as measured using the Morris water maze task of spatial learning.
References:
Oleg V. Dolotov, Ekaterina A. Karpenko, Lyudmila S. Inozemtseva, Tamara S. Seredenina, Natalia G. Levitskaya, Joanna Rozyczka, Elena V. Dubynina, Ekaterina V. Novosadova, Lyudmila A. Andreeva, Lyudmila Yu. Alfeeva, Andrey A. Kamensky, Igor A. Grivennikov, Nikolay F. Myasoedov, Jürgen Engele, Semax, an analog of ACTH(4–10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus, Brain Research, Volume 1117, Issue 1, 2006, Pages 54-60, ISSN 0006-8993, https://doi.org/10.1016/j.brainres.2006.07.108.
Products available for research use only.