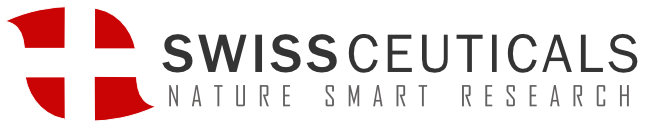ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Introduction
Treatment of neurological injury remains an elusive goal in health care. Despite billions of dollars invested in basic neuroscience and clinical trials, meaningful treatment of many neurological diseases remains a difficult task. As the population ages, certain neurological illnesses such as stroke and dementia will increasingly cause severe neurologic disability. For example, traumatic brain injury (TBI) is the leading cause of death and disability in young people. At an individual level, the overall quality of life of those stricken with these diseases as well as those who care for them is severely affected both financially and emotionally. At the national level, the projected rate of health care spending will be severely affected unless meaningful treatments are discovered and implemented.
Neurorestorative therapy is a concept that is just beginning to be recognized in both the basic science and clinical arena. Neurorestorative therapy treats the intact tissue and not the lesion to invoke a repair of damaged tissues from any type of neurological injury. Neurorestorative therapy acts on intact parenchymal cells, specifically neural progenitor (adult neural stem cells), oligodendrocyte progenitor cells, astroglial cells, and cerebral endothelial cells, to promote neurogenesis, oligodendrogenesis, axonal sprouting, synaptogenesis and angiogenesis, in the injured brain. These restorative processes are associated with improvement in neurological functional outcome.
So… what is considered a neurorestorative therapy? According to the research, it appears that Thymosin Beta-4 peptide may be one of the best available.
What is Thymosin Beta-4 (TB4)?
Tβ4 is a highly conserved and highly expressed 43 amino acid polypeptide with multiple intracellular and extracellular functions. In recent years, the biological function of Tβ4 has received much attention, as it plays an important role in many physiological and pathological activities. Studies show that Tβ4 is closely associated with wound healing, angiogenesis, tumor metastasis, cell apoptosis, corneal repair, and myocardial repair. It is also involved in anti-inflammatory and neurodegenerative processes, especially important for the occurrence and repair of the nervous system.
At present, synthetic Tβ4 has been widely used in research to explore its mechanism of action in different physiological and pathological activities. Studies have found that Tβ4 messenger RNA (mRNA) is widely expressed in mammalian brains such as the hippocampus, dentate gyrus, cerebral cortex, amygdala, and some microglia. It also participates in the differentiation and development of the nervous system after birth, such as synapse generation, neuronal migration, axonal growth, and dendritic plasticity changes, suggesting that it could have neuroprotection, promote axonogenesis and synapse formation properties.
Tβ4 has great potential to promote CNS plasticity and nerve cell regeneration and appears to be a good candidate as a neural repair agent. It can cause neurological recovery in a variety of neurological diseases. Studies have shown that Tβ4 can target multiple nerve cells (including neurons, oligodendrocytes, and microglia) in animal models of nerve injury and can also provide neuroprotection, immunosuppression, and nerve repair such as myelin sheath regeneration, synapse formation, and axonal growth.
Protective effect of Thymosin Beta 4
Tβ4 plays a key role in many cellular processes, including mobility, axon pathfinding, neurite formation, proliferation, and neuron survival.
Samara et al. reported the neuroprotective and neuro repairing effects of Tβ4 on injured nerve cells and the interaction between thymus and thymosin and the nervous and endocrine systems. Exogenous Tβ4 treatment can increase the survival rate of neurons, such as Tβ4 or normal saline given by intraperitoneal injection 3 min or 5 days after spinal cord injury (SCI) in rats in several studies. All behaviors were evaluated, and the number of surviving neurons and oligodendrocytes in Tβ4-treated animals increased significantly.
In addition, in the animal model of traumatic brain injury (TBI), early treatment with intraperitoneal injection of Tβ4 (6 h after injury) reduces the volume of cortical lesions and the loss of hippocampus cells and improves functional recovery, indicating that it is possible to have a neuroprotective effect. Treatment with Tβ4 significantly reduces apoptosis of neural progenitor cells due to oxy-glucose deprivation.
Wirsching et al. analyzed the expression of Tβ4 in chicken (Gallus domesticus) developing cones and overexpressed and knocked down Tβ4 during egg retrovirus transduction and plasmid electroporation. The results indicate the effect of Tb4 on neural stem cell and/or progenitor cell populations. This indicates that Tβ4 has a greater effect on neural stem cell and/or progenitor cell populations.
Choi et al reported that Tβ4 is involved in the control of programmed cell death (PCD) of chicken embryo motor neurons (MNs). Tβ4 can significantly reduce the death of chicken embryo MNs caused by staurosporine (protein kinase C inhibitor). It is suggested that Tβ4-derived peptides can be used for anti-apoptotic treatment of neuropathology related to neuronal apoptosis.
Popoli et al. also reported that Tβ4 could reduce the toxic effect of glutamate on primary cultured cortical neurons, significantly reduce neuronal apoptosis, and protect neurons in rat models of excitatory amino acid damage induced by kainic acid.
Morris et al. found that Tβ4 could promote the migration and differentiation of neural stem cells from the subventricular zone (SVZ) to ischemic lesions and reduce brain function damage after cerebral ischemia in adult rats, suggesting that Tβ4 may achieve these effects by stimulating neuron migration and inducing the expression of extracellular matrix.
According to the latest report, based on Tβ4 potential anti-inflammatory molecules and neuroprotective and myelin regeneration molecules and their mechanism of action, it is conceivable to use Tα1 (thymosin-α1) and Tβ4 alone in multiple sclerosis (MS) or with other approved possible application of drug combination therapy.
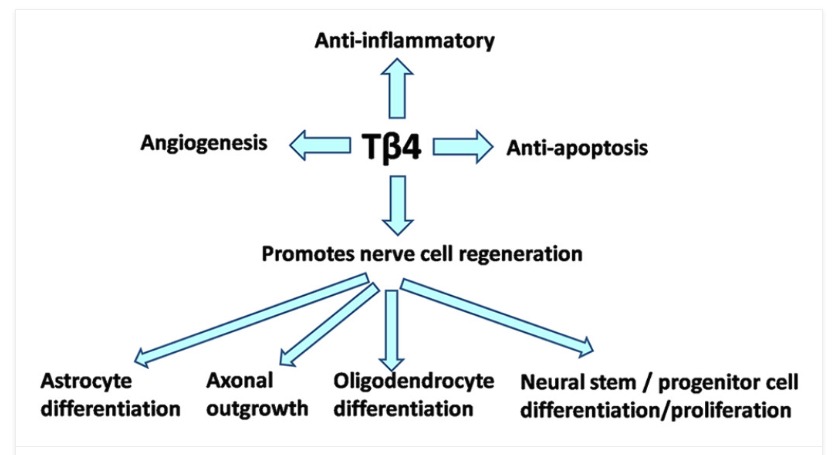
Additional Benefits based on Research and the Literature
Thymosin Beta-4 has been shown to:
- Sports/athletic injury
- Soft tissue repair
- Tendon/ligament/muscle repair
- Pressure ulcers / venous stasis ulcers
- Immune support (as monotherapy or in conjunction with Thymosin alpha 1)
- Brain issues if autoimmunity suspected
- Multiple sclerosis
- Ischemic stroke
- Spinal cord injuries
- TBI; concussion support (in conjunction with BPC 157)
- Sepsis
- Dry eye disorders
- Ocular tissue injuries including corneal wound healing and repair
- Chemical burns
- Diabetes
- Corneal transplants
- Cardioprotective
- NAFLD – non-alcoholic fatty liver disease
- Lung inflammation / fibrosis
- May improve hair growth
Research Example #1:
Treatment of neurological injury with thymosin β4
Neurorestorative therapy targets multiple types of parenchymal cells in the intact tissue of the injured brain tissue to increase neurogenesis, angiogenesis, oligodendrogenesis, and axonal remodeling during recovery from neurological injury. In our laboratory, we tested thymosin β4 (Tβ4) as a neurorestorative agent to treat models of neurological injury. This review discusses our results demonstrating that Tβ4 improves neurological functional outcome in a rat model of embolic stroke, a mouse model of multiple sclerosis, and a rat model of traumatic brain injury. Tβ4 is a pleiotropic peptide exhibiting many actions in several different types of tissues. One mechanism associated with improvement of neurological improvement from Tβ4 treatment is oligodendrogenesis involving the differentiation of oligodendrocyte progenitor cells to mature myelin-secreting oligodendrocytes. Moreover, our preclinical data provide a basis for movement of Tβ4 into clinical trials for treatment of these devastating neurological diseases and injuries.
Research Example #2:
Treatment of traumatic brain injury with thymosin β4 in rats
This study was designed to investigate the efficacy of delayed thymosin β4 (TB4) treatment of traumatic brain injury (TBI) in rats.
Young adult male Wistar rats were divided into the following groups: 1) Sham group (6 rats); 2) TBI + Saline group (9 rats); 3) and TBI + Tβ4 group (10 rats). TBI was induced by controlled cortical impact over the left parietal cortex. Thymosin β4 (6 mg/kg) or saline was administered intraperitoneally starting at Day 1 and then every 3 days for an additional 4 doses. Neurological function was assessed using a modified neurological severity score (mNSS), foot fault and Morris water maze tests. Animals were killed 35 days after injury, and brain sections stained for immunohistochemistry to assess angiogenesis, neurogenesis, and oligodendrogenesis after Tβ4 treatment.
Compared to the saline treatment, delayed Tβ4 treatment did not affect lesion volume but significantly reduced hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, increased oligodendrogenesis in the CA3 region, and significantly improved sensorimotor functional recovery and spatial learning.
These data for the first time demonstrate that delayed administration of Tβ4 significantly improves histological and functional outcomes in rats with TBI, indicating that Tβ4 has considerable therapeutic potential for patients with TBI.
Research Example #3
Protective effect of Tβ4 on central nervous system tissues and its developmental prospects
Tissue repair and regeneration in the central nervous system (CNS) remains a serious medical problem. CNS diseases such as traumatic and neurological brain injuries have a high mortality and disability rate, thereby bringing a considerable amount of economic burden to society and families. How to treat traumatic and neurological brain injuries has always been a serious issue faced by neurosurgeons. The global incidence of traumatic and neurological brain injuries has gradually increased and become a global challenge. Thymosin β4 (Tβ4) is the main G-actin variant molecule in eukaryotic cells. During the development of the CNS, Tβ4 regulates neurogenesis, tangential expansion, tissue growth, and cerebral hemisphere folding. In addition, Tβ4 has anti-apoptotic and anti-inflammatory properties. It promotes angiogenesis, wound healing, stem/progenitor cell differentiation, and other characteristics of cell migration and survival, providing a scientific basis for the repair and regeneration of injured nerve tissue. This review provides evidence to support the role of Tβ4 in the protection and repair of nervous tissue in CNS diseases, especially with the potential to control brain inflammatory processes, and thus open up new therapeutic applications for a series of neurodegenerative diseases.
Overall Conclusion
Many previously published studies indicate that Tβ4, a new type of multifunctional bioactive molecule, plays a crucial role in neuroprotection and nerve repair in the treatment of traumatic and neurological brain injuries.
As shown in previous research as well, the most important activity of Tβ4 may be its actin-binding properties to promote the migration and differentiation of neural stem/progenitor cells in the injured area, thereby promoting brain repair or regeneration processes, and can be used as a potential nerve repair, thus, it has therapeutic potential for various nervous system injuries and neurodegenerative diseases.
Tβ4 may be a promising therapeutic approach to reduce neuroinflammation and reduce symptoms associated with the pathogenesis of progressive psychosis and neurodegenerative diseases.